Table of contents
- Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: a randomized-controlled clinical trial
- VitaminDWiki -
22 studies in both categories Virus and Loading Dose - VitaminDWiki – COVID-19 treated by Vitamin D - studies, reports, videos
Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: a randomized-controlled clinical trial
Trials volume 24, Article number: 123 (2023)
Hela Abroug, Amani Maatouk, Cyrine Bennasrallah, Wafa Dhouib, Manel Ben Fredj, Imen Zemni, Meriem Kacem, Salma Mhalla, Sarra Nouira, Manel Ben Belgacem, Aymen Nasri, Rim Klii, Chawki Loussaief, Nissaf Ben Alya, Ines Bouanene & Asma Belguith Sriha
Introduction
The present study aimed to determine the impact of vitamin D supplementation (VDs) on recovery delay among COVID-19 patients.Methods
We performed a randomized controlled clinical trial at the national COVID-19 containment center in Monastir (Tunisia), from May to August 2020. Simple randomization was done in a 1:1 allocation ratio. We included patients aged more than 18 years who had confirmed reverse transcription-polymerase chain reaction (RT-PCR) and who remained positive on the 14th day. The intervention group received VDs (200,000 IU/1 ml of cholecalciferol); the control group received a placebo treatment (physiological saline (1 ml)). We measured the recovery delay and the cycle threshold (Ct) values in RT-PCR for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The log-rank test and hazard ratios (HR) were calculated.Results
A total of 117 patients were enrolled. The mean age was 42.7 years (SD 14). Males represented 55.6%. The median duration of viral RNA conversion was 37 days (95% confidence interval (CI): 29–45.50) in the intervention group and 28 days (95% CI: 23–39) in the placebo group (p=0.010). HR was 1.58 (95% CI: 1.09–2.29, p=0.015). Ct values revealed a stable trend over time in both groups.Conclusion
VDs was not associated with a shortened recovery delay when given to patients for whom the RT-PCR remained positive on the 14th day.Trial registration
This study was approved by the Human Subjects Protection Tunisia center (TN2020-NAT-INS-40) on April 28, 2020, and by ClinicalTrial.gov on May 12, 2021 with approval number ClinicalTrials.gov ID: NCT04883203.
Download the PDF from VitaminDWiki
VitaminDWiki -
22 studies in both categories Virus and Loading Dose This list is automatically updated
- Single 600,000 IU dose of nanoemulsion Vitamin D is safe and effective to fight COVID, even if delay until enter ICU – RCT May 2024
- COVID recovery 1.6X faster after 200,000 IU of Vitamin D RCT – Feb 2023
- Treat COVID with early high-dose Vitamin D (20th as of June 2022)
- 400,000 IU of vitamin D 3 days after COVID symptoms reduced 14 day mortality by 3X – Annweiler RCT May 2022
- COVID patients not helped by 500K IU of Vitamin D (they already had enough) – May 2022
- Loading dose of Vitamin D for patients hospitalized with COVID (140,000 IU) – RCT completed 2021
- COVID Ventilation 2X less likely if 200,000 IU of Vitamin D when enter hospital – May 2022
- FLCCC COVID guidelines now include vitamin D loading doses - Jan 2022
- Take lots of Vitamin D at first signs of COVID
- Rapid Vitamin D Delivery May Result in Better COVID Outcomes - Dec 9, 2021
- French recommended 200,000 IU of Vitamin D to stop COVID-19 - Jan 2021
- COVID-19 mortality reduced 4X (chart looks like 2X) by large, infrequent doses of Vitamin D in France – July 2021
- 600,000 IU of Vitamin D helped 26 out of 28 COVID-19 patients in ICU (Brazil and Bolivia) June 2021
- Infectious Mononucleosis (virus) and Vitamin D - many studies
- Those getting high dose vitamin D were 7 X less likely to die of COVID-19 - Dec 11, 2020
- COVID-19 Vitamin D Overview - Sunil video and transcript - Dec 8, 2020
- Vitamin D has eliminated ICU COVID-19 in hospital in Dubai since June - Sept 26, 2020
- Severe COVID-19 not fought by vitamin D when given too late - RCT Nov 18, 2020
- COVID-19 defeated 3x faster by 420,000 IU Vitamin D nanoemulsion – RCT Nov 12, 2020
- French National Academy recommended 100,000 IU of Vitamin D to elderly to fight COVID-19 - May 2020
- Residents of a Nursing Home who choose monthly Vitamin D had 4X fewer COVID-19 deaths – Nov 2, 2020
- Cerebral malaria deaths prevented by loading dose of vitamin D (mice) – Sept 2018
VitaminDWiki – COVID-19 treated by Vitamin D - studies, reports, videos
As of March 31, 2024, the VitaminDWiki COVID page had: trial results, meta-analyses and reviews, Mortality studies see related: Governments, HealthProblems, Hospitals, Dark Skins, All 26 COVID risk factors are associated with low Vit D, Fight COVID-19 with 50K Vit D weekly Vaccines Take lots of Vitamin D at first signs of COVID 166 COVID Clinical Trials using Vitamin D (Aug 2023) Prevent a COVID death: 9 dollars of Vitamin D or 900,000 dollars of vaccine - Aug 2023
5 most-recently changed Virus entries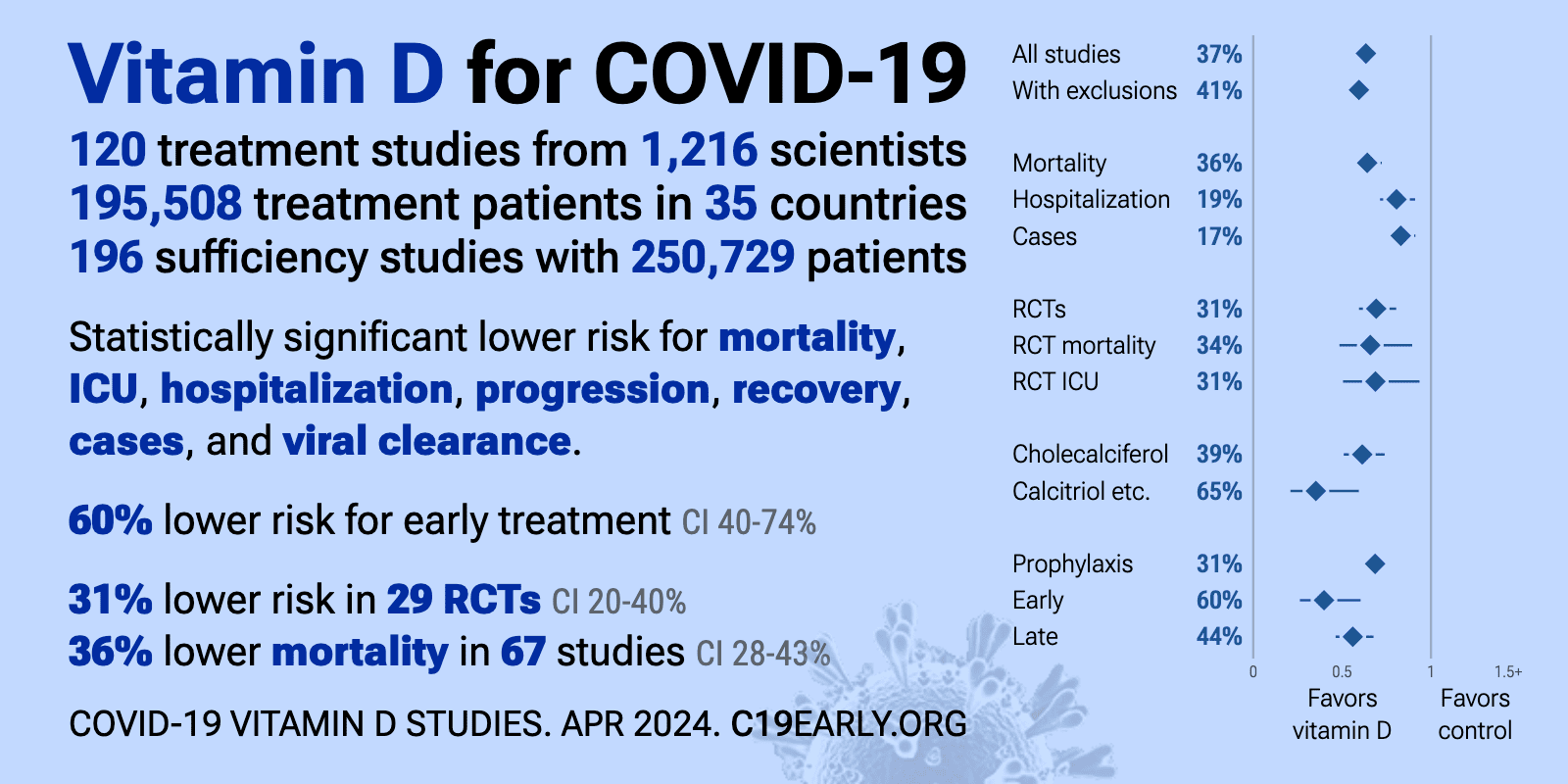
- The above image is automatically updated
Printer Friendly Follow this page for updates2377 visitors, last modified 22 Feb, 2023,