International Society for Nutritional Psychiatry Research Practice Guidelines for Omega-3 Fatty Acids in the Treatment of Major Depressive Disorder
Psychotherapy and Psychosomatics, DOI: 10.1159/000502652
Ta-Wei Guua b David Mischoulonc Jerome Sarris e Joseph Hibbelnf Robert K. McNamarag Kei Hamazakih Marlene P. Freeman1 Michael Maesj Yutaka J. Matsuokak R.H. Belmaker' Felice Jackam Carmine Parianten Michael Berko Wolfgang Marxm Kuan-Pin Su
Depression category listing has 271 items along with related searches
Pages listed in BOTH the categories Depression and Omega-3
-
Overweight needed more EPA (4 grams) to fight depression – RCT Aug 2022
-
Anxiety, depression, and suicide have recently surged (Note: Vitamin D, Omega-3, and Magnesium help) – May 2022
-
Omega-3 did not prevent depression (they failed to reduce Omega-6, which blocks Omega-3) – RCT Dec 2021
-
Mental health not helped by vitamin D monotherapy (adding Omega-3 and Magnesium help) – review Nov 2021
-
Benefits of Omega-3 plus Vitamin D were additive – RCT Sept 2021
-
Depression treatments: diet, exercise, bright light, Vitamin D, B12, Omega-3, Zinc, Music, etc. – May 2019
-
Omega-3 helps treat Major Depression – International Consensus Sept 2019
-
Mental disorders fought by Omega-3 etc. - meta-meta-analysis Oct 2019
-
Omega-3 reduces Depression. Anxiety, Stress, PTSD, etc. – Aug 2018
-
Depression treated by Omega-3 (again) – meta-analysis Aug 2019
-
Depression after childbirth 5 X less likely if good Omega-3 index – April 2019
-
Occupational burnout reduced after 8 weeks of Omega-3 – RCT July 2019
-
Anxiety severity reduced if more than 2 grams of Omega-3 – meta-analysis Sept 2018
-
Psychotic disorders not treated by Omega-3 when patents take anti-depressants and get therapy – June 2018
-
Happy Nurses Project gave Omega-3 for 3 months – reduced depression, insomnia, anxiety, etc for a year – RCT July 2018
-
Depression – is it reduced by Vitamin D and or Omega-3 – RCT 2019
-
Benefits of Omega-3 beyond heart health - LEF Feb 2018
-
Omega-3 improves gut bacteria, reduces inflammation and depression – Dec 2017
-
Unipolar depression treated by Omega-3, Zinc, and probably Vitamin D – meta-analysis Oct 2017
-
Omega-3 reduces many psychiatric disorders – 2 reviews 2016
-
Omega-3 does not consistently treat depression if use small amounts for short time period – review Oct 2016
-
How Omega-3 Fights Depression – LEF July 2016
-
Depression due to inflammation reduced by Omega-3 (children and pregnant) – Nov 2015
-
Depression treated somewhat by Omega-3 (St. John's Wort better) – RAND org reviews 2015
-
Depression substantially decreased with Omega-3 – Sept 2015
-
Omega-3 for just 3 months greatly reduced psychosis for 80 months – RCT Aug 2015
-
Omega-3 prevents PTSD and some mood disorders - Aug 2015
-
Omega-3, Vitamin D, and other nutrients decrease mental health problems – March 2015
Meta-analyses of Vitamin D and Depression
-
Depression reduced by 8,000 IU of Vitamin D daily – meta-analysis Nov 2024
-
Depression 1.6 X more likely if low Vitamin D, taking Vitamin D reduces depression – umbrella of meta-analyses – Jan 2023
-
Depression in seniors greatly reduced by Vitamin D (50,000 IU weekly) – meta-analysis June 2023
-
Depression reduced if take more than 5,000 IU of vitamin D daily – umbrella meta-analysis – Jan 2023
-
Depression reduced if use more than 2,800 IU of vitamin D – meta-analysis Aug 2022
-
Depression is treated by 2,000 IU of Vitamin D – 2 meta-analyses July 2022
-
Depression treated by 50K IU Vitamin D weekly (but not 1,000 IU daily) – meta-analysis Jan 2021
-
Mental disorders fought by Omega-3 etc. - meta-meta-analysis Oct 2019
-
Depression less likely if more Vitamin D (12 percent per 10 ng) – meta-analysis July 2019
-
Anxiety severity reduced if more than 2 grams of Omega-3 – meta-analysis Sept 2018
-
Less depression in seniors taking enough Omega-3 – meta-analysis July 2018
-
Unipolar depression treated by Omega-3, Zinc, and probably Vitamin D – meta-analysis Oct 2017
-
Depression is associated with low Magnesium – meta-analysis April 2015
-
Clinical Trials of vitamin D can have “biological flaws” – Jan 2015
-
Slight depression not reduced by adding vitamin D if already had enough (no surprise) – meta-analysis – Nov 2014
-
Anti-depression medication about as good as big increase in vitamin D – meta-analysis of flawless data April 2014
-
Depression might be reduced by vitamin D – meta-analysis March 2014
-
Low vitamin D and depression - Study and meta-analysis, April 2013
-
2X more likely to be depressed if low vitamin D (cohort studies) - Meta-analysis Jan 2013
Download the PDF from VitaminDWiki
Table 1. ISNPR practice guidelines for n–3 PUFAs in the treatment of MDD
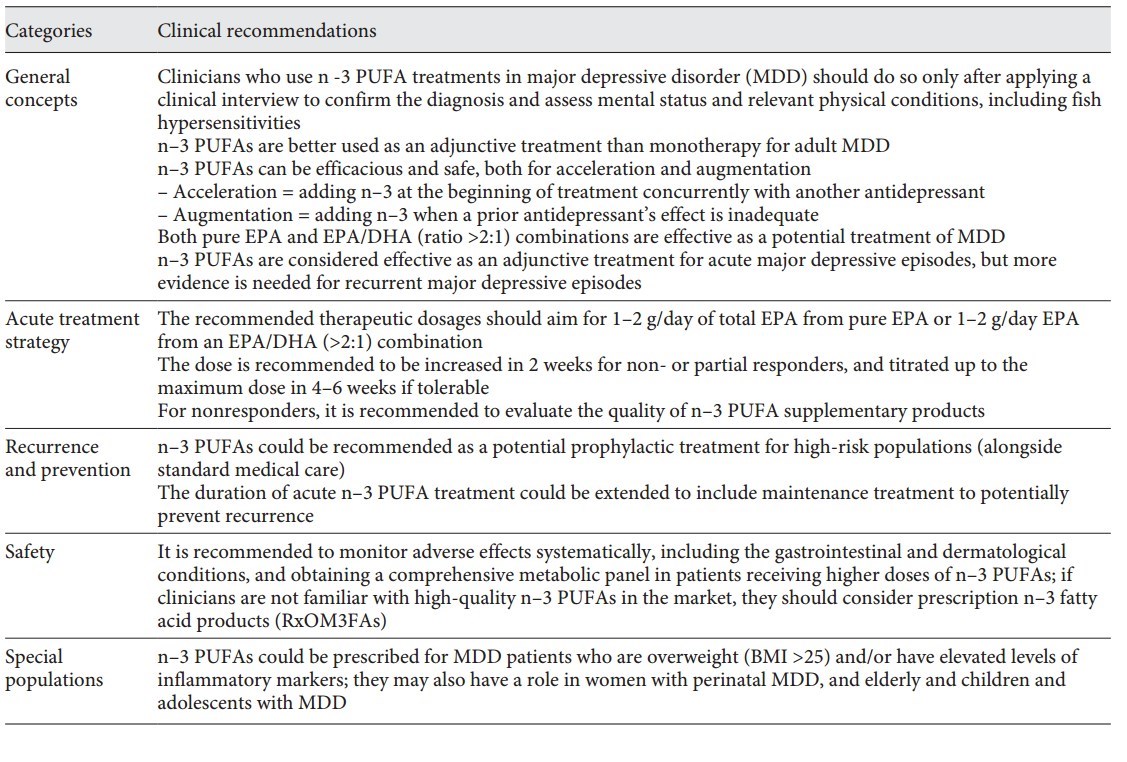
Major depressive disorder (MDD) is a complex mental illness with unmet therapeutic needs. The antidepressant effects of w-3 polyunsaturated fatty acids (n-3 PUFAs) have been widely reported. The subcommittee of the International Society for Nutritional Psychiatry Research organized an expert panel and conducted a literature review and a Delphi process to develop a consensus-based practice guideline for clinical use of n-3 PUFAs in MDD. The guideline focuses on 5 thematic areas: general concepts, acute treatment strategy, depression recurrence monitoring and prevention, use in special populations, and potential safety issues. The key practice guidelines contend that: (1) clinicians and other practitioners are advised to conduct a clinical interview to validate clinical diagnoses, physical conditions, and measurement-based psychopathological assessments in the therapeutic settings when recommending n-3 PUFAs in depression treatment; (2) with respect to formulation and dosage, both pure eicosapentaenoic acid (EPA) or an EPA/docosahexaenoic acid (DHA) combination of a ratio higher than 2 (EPA/DHA >2) are considered effective, and the recommended dosages should be 1-2 g of net EPA daily, from either pure EPA or an EPA/DHA (>2:1) formula; (3) the quality of n-3 PUFAs may affect therapeutic activity; and (4) potential adverse effects, such as gastrointestinal and dermatological conditions, should be monitored, as well as obtaining comprehensive metabolic panels. The expert consensus panel has agreed on using n-3 PUFAs in MDD treatment for pregnant women, children, and the elderly, and prevention in high-risk populations. Personalizing the clinical application of n-3 PUFAs in subgroups of MDD with a low Omega-3 Index or high levels of inflammatory markers might be regarded as areas that deserve future research.
Portions of PDF
Introduction
Major depressive disorder (MDD) affects one tenth of the population and has been the world’s leading cause of disability [1, 2]. MDD is of heterogeneous etiology with multiple contributory biological mechanisms. Pharmacological treatments with the currently available antidepressants, although proven to be effective in treating moderate to severe symptoms in MDD, have only modest effect sizes but various adverse effects [3]. Therefore, to optimize the patients’ outcomes, clinicians need more efficacious and tolerable treatments supported by valid scientific evidence and reliable practice guidelines.
Omega-3 polyunsaturated fatty acids (n-3 PUFAs), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) have drawn clinical attention of medical specialties [4-7]. Several lines of evidence have suggested the efficacy of n-3 PUFAs as a preventive and treatment strategy in MDD, from epidemiological and case-controlled studies [8, 9] to randomized-controlled trials [10-20] and meta-analyses [21-30]. In addition to clinical studies that examine the efficacy [31, 32] and tolerability [33], the mechanisms of n-3 PUFAs’ antidepressant effects have also been rigorously studied. Several key mechanisms have been proposed, including neuronal cell plasticity and neurogenesis, neurotransmitter dysregulation, and neuro-inflammation [6, 34, 35].
Despite the clinical and biological evidence, and empirical experience using n-3 PUFAs as an alternative or adjunctive treatment for MDD, there is a current lack of definitive clinical practice guidelines to assist clinicians in the prescriptive application of n-3 PUFAs for MDD. To address this, an advisory subcommittee from the International Society for Nutritional Psychiatry Research (ISNPR) was formed to provide international consensus- based practice guidelines for the evidence-based prescriptive use of n-3 PUFAs for the treatment of MDD.
Methodology
Overview
The format and content of this practice guideline for n-3 PUFAs were developed through 3 stages, including (1) conducting a literature review on major research findings of n-3 PUFAs in MDD treatment; (2) generating a clinical guideline questionnaire and obtaining consensus through a 2-round, web-based modified mini-Delphi survey; and (3) optimizing the guidelines from consensus meetings with international experts.
Literature Review and Search Strategy
We had the first preparatory meeting held by a subcommittee under the auspices of 7 key members from the ISNPR in November 2017. ISNPR is an international collaboration of academics advocating recognition of various nutrients as crucial determinants of both physical and mental health, and the positional statements had been published elsewhere [36, 37].
A systematic review of published literature was conducted with an emphasis on randomized controlled trials (RCTs), systematic reviews and meta-analyses, focusing on the quality of evidence and the implications. Observational studies, physiological or preclini- cal studies in nonhuman subjects, and ongoing trials would also be reviewed as only supportive evidence.
The subsequent literature review was conducted via PubMed and from the Cochrane Database from inception to May 29, 2019, by the terms “omega-3 polyunsaturated fatty acids,” “n-3 PUFAs,” “major depressive disorder,” and “treatment.” Articles retrieved from these searches and relevant references cited in those articles were also reviewed. High-quality articles published in English, Chinese, and Japanese were included for preliminary review. The outline of the review was drafted by 4 core authors (T.G., D.M., J.S., and K.-P.S.), and then all the authors examined cross-referencing, identified missing important studies, revised the draft of the review, and discussed through subsequent online conferences.
The evidence was categorized using a modified format of the 2011 Levels of Evidence grading system developed by the Oxford Center for Evidence-Based Medicine. Level 1 evidence derives from meta-analyses and systematic reviews of narrow confidence interval (CI) or little conflicting results. Level 2 evidence includes appropriately designed RCTs. Smaller and lower-quality RCTs and nonrandomized controlled cohort/follow-up studies are considered level 3 evidence. Case reports and case series were considered and discussed in the expert consensus meeting to formulate level 4 evidence.
Guideline Questionnaire and Delphi Survey
Four core authors (T.G., D.M., J.S., and K.-P.S.) had an initial online meeting in September 2018 to confirm the evidence obtained from the review and to discuss the major themes and prescriptive issues that could be assessed using a survey (and codified in a resultant consensus guideline). Previous guidelines about n-3 PUFAs and MDD, including using n-3 PUFAs in the treatment of cardiovascular diseases by the American Heart Association, were included as references and discussed to facilitate the formation of the questionnaire [38-40]. After internal correspondences, the draft questionnaire consisted of 20 questions covering 5 major themes: (1) general concepts, (2) acute treatment strategy, (3) depression recurrence monitoring and prevention, (4) special populations, and (5) safety issues.
A Likert scale (0-10) was used for the 19 closed questions (0 = fully against, 10 = fully agree). Questions that scored a predefined consensus level (7.0/10) were included into the guidelines, questions that scored between 5.1 and 6.9 were discussed while questions with scores lower than 5.0 were allocated into a proposed second round Delphi survey with inverse questions. The questionnaire was then tabulated in a web-based survey platform (Survey Monkey) for online voting.
Consensus Meetings
There were 14 experts who participated in the Delphi survey conducted in October 2018. The panellists were selected mainly according to the h-index of the Web of Science database, under the topic of “depression and omega-3” with time span until the end of September 2018 and completed with purposive snowball sampling among active ISNPR members in this topic.
In the online consensus meetings, the panellists focused on confirming the affirmed statements for inclusion and discussing the 3 questions that did not reach the consensus level. After the meeting, the results from the literature review, together with recommendations and the feedback from the survey and meeting, were synthesized into the final version of the official practice guidelines.
Results
Diagnosis and Measurement-Based Care
The panel consensus emphasizes the importance on diagnosis-based clinical interview and measurement- based depression assessments to validate the accurate diagnosis and psychopathology severity of MDD in the clinical setting [41]. Indeed, when the antidepressant efficacy of n-3 PUFAs is rigorously studied, the pooled effects favor n-3 PUFAs over placebo mainly in RCTs with clinician interview-based diagnosis, rather than in trials including subjects with subclinical depressive symptoms screened by questionnaire [23, 29].
Disease Stages
Acute Stage of MDD
The efficacy of small to moderate effect sizes with good safety profiles has been reported in several well-designed RCTs with MDD patients in the acute stage [10-12, 20]. Further, the pooled results from the RCTs for MDD adults (nonpregnant and no-children populations) support the use of n-3 PUFAs for “adjunctive” treatment rather than n-3 PUFA monotherapy (level 1) [24, 25, 30, 42, 43].
Recurrent MDD and Maintenance Treatment
Patients with MDD usually require maintenance treatment after the acute depressive episode. However, we identified no studies having enough follow-up duration to address the prevention effects for recurrent MDD. Therefore, current evidence is not adequate to either support or refute the prescription of n-3 PUFAs as longer- term maintenance therapy or for recurrent major depressive episodes. However, considering that most antidepressant medication might be associated with higher dropout rates as compared to placebo [3], the advantage of tolerability profiles of n-3 PUFAs might suggest its potential in maintenance treatment (level 2).
Treatment Strategy (Monotherapy, Augmentation, or Acceleration)
The panel endorses the application for augmentative therapy of n-3 PUFAs because cumulative evidence favors the efficacy of n-3 PUFAs over placebo with the enhanced antidepressant effects clearly shown in meta-analyses (level 1) [24, 25, 30, 42, 43]. Furthermore, in one metaanalysis investigating the time points when n-3 PUFAs were added to antidepressants [42], the results showed beneficial effects in either adding n-3 PUFAs at the beginning of the treatment (as an accelerating agent) or adding them at the time when the standing antidepressant was inadequate (i.e., as an augmentative agent; level 1).
Our literature review revealed only a few studies assessing n-3 PUFAs as a monotherapy for MDD. Monotherapy with n-3 PUFAs may have therapeutic benefits in children [44] and pregnant women [14] with depression. However, Mischoulon et al. [45] revealed that neither EPA nor DHA monotherapy were superior to placebo in MDD adults. In another study, patients with post- myocardial infarction were treated with 460 mg EPA and 380 mg DHA for 12 months as monotherapy, and there was also no significant effect in favor of n-3 PUFAs [46]. Therefore, current evidence is inadequate overall of using n-3 PUFAs as monotherapy for MDD, and further RCTs are needed to provide support for or against the use of n-3 PUFAs as a monotherapy for adult MDD (nonpregnant and no-children) patients (level 2).
Table 1. ISNPR practice guidelines for n-3 PUFAs in the treatment of MDD (at top of page)
Dosing, Ratio, and Duration
The functional status of n-3 PUFAs in an individual can be influenced by many factors like dietary patterns, gender, age, and metabolic functions. Therefore, it is impossible to predict the absolute effects of a fixed dose n-3 PUFA supplementation in MDD individuals. In addition, there is no RCT investigating clinical dosing of n-3 PUFAs based on individual dietary or blood level differences.
According to the available RCT results, the panel advises that the starting dose should be at least 1 g of net EPA in a pure EPA form or in an EPA/DHA combination (ratio higher than 2). If well tolerated, the dose could be titrated up to at least 2 g of net EPA daily in 2-4 weeks for partial responsiveness (level 1) [21,22, 24, 25, 27-30, 42, 43]. The panel also reaches a unanimous consensus on the recommendation of EPA/DHA ratio of >2:1 to be crucial to n-3 PUFAs’ antidepressant effects (level 1). Indeed, this conclusion is supported by most of the meta-analyses that the higher ratio of EPA, the better the therapeutic outcome, while DHA as main component had no detectable pooled effects on the MDD symptoms [21,23-25, 29].
There was insufficient data to conclude the optimal duration of n-3 PUFA supplementation. The duration of most RCTs varies from 4 to 16 weeks. Due to the length of time needed for n-3 PUFAs to be incorporated into brains, and for the downstream neuroplastic effects and anti-inflammatory actions, the panel endorses a prescriptive guideline of at least 8 weeks. Clinicians are advised to check the quality of the n-3 PUFA prescription of nonresponders, and evaluate the response and the tolerability profiles for an optimal duration (level 2) [24, 25, 27].
Safety and Adverse Effects
One recent meta-analysis comprehensively examined the adverse effects reported when using prescribed n-3 PUFA products in various clinical populations. n-3 PUFAs were found to be associated with higher rates of mild gastrointestinal symptoms (such as fishy taste, belching, and nausea), less in skin abnormalities (such as eruption and itchiness), and no serious adverse effects [33]. Another meta-analysis using adverse effects as one of the primary outcomes reported that, despite poor data quality, the numbers of individuals experiencing adverse events were similar in intervention and placebo groups and were negligible (level 1) [30]. The risk of theoretical adverse effects of excessive bleeding did not exist, and current evidence suggests that under concurrent usage of antiplatelet or anticoagulant agents, doses up to 4 g of n-3 PUFAs daily are not associated with an increased risk of major bleeding [47]. Although both a recent meta-analysis focusing on pregnancy [48] and the other focusing on perioperative periods [49] support similar safety profiles compared with placebo in general and in bleeding-related adverse events, and most in vivo studies do not suggest that n-3 PUFAs would impact either platelet aggregation or adhesion in healthy subjects [50], we recommend shared decision-making discussions between patients and clinicians on bleeding-related hematological examinations, especially if the patient has underlying fibrinogen dysfunction or is taking antiplatelet or anticoagulant agents.
Laboratory measurements have revealed that n-3 PUFAs (compared to placebo) may cause higher fasting blood sugar, glutamate pyruvate transaminase, low-density lipoprotein cholesterol and blood urea nitrogen, and lower hemoglobin and hematocrit levels. Although the mean differences were relatively small, and may have only statistical rather than clinical importance, the panel recommends that, when using higher doses, these biomarkers may need to be monitored (level 1). It is interesting to note that in this meta-analysis, combining EPA and DHA was associated with higher rates of the aforementioned adverse effects, compared with EPA alone [33]. The monitoring profiles are summarized in Table 2.
Discussion
To our knowledge, this is the first international research society consensus-based practice guideline for clinical use of n-3 PUFAs in MDD. The guideline includes 12 clinical recommendations and is well summarized in Table 1 based on the items which reached consensus levels. In general, the recommendations focus on 5 thematic areas: general concepts, acute treatment strategy, recurrence and prevention, special populations, and safety. The guideline did not include, yet discussed in the previous section Results, the 3 items of our expert survey that did not reach consensus levels.
The panel emphasizes the importance of accurate clinical diagnosis and measurement-based psychopathological assessments practised in the therapeutic settings when recommending n-3 PUFAs in depression treatment. As cumulative evidence favors the efficacy of n-3 PUFAs as an adjunctive use on top of antidepressant medication, the attitude of prescribing n-3 PUFAs should not be based on antimedication.
As meta-analyses reveal only small but statistically significant effects, there are always critical debates on the benefits of n-3 PUFAs for MDD. The estimated effect sizes (standardized mean differences between n-3 PUFAs and placebo) range from 0.23 to 0.56 (with wide CI) in treating DSM-defined MDD patients in 3 recent metaanalyses [24, 29, 30]. However, small effect sizes are also reported for all kinds of antidepressant drugs compared to placebo, with the standardized mean differences between 0.30 and 0.47 (with narrower CI) [3, 66, 67]. Till now, the scientific evidence and clinical opinions do not support any particular treatment based on efficacy differences. Although n-3 PUFAs would not be regarded as “a new antidepressant,” when the clinicians consider taking the differences in adverse events and patients’ preference into consideration, n-3 PUFAs would be potentially an applicable alternative choice of treatment [68].
Three practical strategies could be considered for the unmet needs in depression treatment as every single recommended therapy only has small effects: “an open- minded attitude to integrative intervention,” “the application of personalized medicine,” and a “shared decision making process based on balanced information to enhance treatment adherence.”
First, the integrative intervention is often criticized for overpromotion on efficacy and safety and for nonregulation of product quality and malpractice by trustworthy authorities. This panel consists of the most cited experts in the fields and aims to provide an evidence-based empirical practice guideline to help clinicians to have a more open-minded attitude to recommend n-3 PUFAs to depressed patients. For other “nonpatentable” interventions with sound clinical and preclinical evidence (e.g., exercise [69], mindfulness [70, 71], or acupuncture [72]), we need more well-established expert consensus and practice guidelines supported by trustworthy authorities for the same reasons.
The second important strategy to increase the treatment effects is subtyping patients into more homogeneous subgroups, as MDD populations can be extremely heterogenous based on current diagnostic systems [73]. Rapaport et al. [18] published an interesting RCT and demonstrated that EPA monotherapy was more effective than the placebo or DHA in patients with elevated markers of inflammation. Specifically, the overall treatment group differences were negligible (ES = -0.09 to -0.13) among MDD patients without stratifying. However, subjects classified with “high” inflammation improved more on EPA than placebo (ES = -0.39 to -1.11 from any one marker to 4-5 markers) or DHA (ES = -0.60 to -1.10 from any one marker to 4-5 markers). Furthermore, EPA-placebo separation increased with increasing numbers of markers of high inflammation. In other words, employing biomarkers of inflammation indeed facilitated identification of a more homogeneous cohort of subjects with MDD who would respond better to n-3 PUFA antidepressant treatment [32]. Therefore, this current practice guideline specifies subgroups of MDD for n-3 PUFAs, including perinatal depression, childhood depression, and MDD with low-grade inflammation or comorbid obesity.
Finally, it has been noted that a shared decision-making process may enhance patients’ adherence and satisfaction to treatment, especially in chronic illnesses [74]. As MDD is often a long-lasting, relapse-remitting illness, assessing the patient’s attitudes toward treatment, integrating information regarding responsiveness, vulnerability, comorbidity and important psychosocial and contextual factors, rather than providing a reductionistic view of treatment options, may allow the patients to actively participate in the therapeutic settings and subsequently improve the adherence and satisfaction toward the treatment [75-78]. In this guideline, we tried to address all the relevant clinical aspects when considering n-3 PUFAs for MDD patients, and it is important to emphasize that “the process” of discussing the information can be as important as the information itself.
Despite several meta-analyses and independent clinical trials which have shown that n-3 PUFAs were more effective than placebo, the meta-analyses from 2 groups failed to detect antidepressant effects of n-3 PUFAs [22, 79]. In fact, the main reasons of inconsistent results from metaanalyses in n-3 PUFA trials are due to common methodological flaws such as pooling heterogeneous clinical trials, applying nonstandardized diagnostic procedures and unreliable outcome measurements, and implementing improper intervention methods [29, 80], which are rarely found in high-quality clinical trials conducted by pharmaceutical companies. In the first meta-analysis with negative findings [22], the authors included a clinical trial [81] enrolling individuals according to self-rating scales, but not structured interview, and in nonclinical settings that weighted up to 31.7% of the whole pooled estimation. In another meta-analysis showing negative outcomes [79], one single 43.3%-weighted clinical trial was included [82], which not only applied no appropriate tools for clinical diagnoses or assessments, but also defined its n-3 PUFA intervention as “advising” subjects to “eat more fish.” Obviously, the methodological flaws undermined the possibility to detect the small signals in clinical trials for n-3 PUFAs’ antidepressant effects. And if the problematic RCTs were taken away, all the meta-analyses from independent groups with well-conducted designs showed positive results [23-25, 27, 29, 83].
Some limitations need to be taken into account when applying this guideline in clinical settings. First, 3 items did not reach consensus levels: (1) “n-3 PUFAs are one of the potential monotherapies for adult MDD”; (2) “pure EPA is more recommended than EPA/DHA (>2) combination”; and (3) “n-3 PUFAs are considered similarly effective for recurrent MDD.” Therefore, we concluded that the evidence is inadequate overall of using n-3 PUFA prevention of MDD recurrence or as a monotherapy. In addition, the consensus describes that “the pure EPA form or the EPA/DHA combination could both be effective.” Second, we should be aware of the aggregated mild treatment effects in meta-analyses and the inconsistence in some of the RCTs. In addition to general methodological flaws mentioned previously, the other main reasons for inconsistent findings from several RCTs include insufficient statistic powers due to small sample sizes, poor quality of product preparations, and inadequate dosage or ratio of EPA in the n-3 PUFA supplementations. It is very important to note that the clinical trials using DHA-predominant formulations are consistently ineffective [55, 56, 81, 84-87]. Therefore, this guideline includes: “Both pure EPA and EPA/DHA (ratio >2:1) combinations are effective as a potential treatment of MDD (item 4).” Third, unlike medication, most n-3 PUFA supplementary products are not strictly regulated by government regulation. Therefore, this guideline recommends that “If clinicians are not familiar with high-quality n-3 PUFAs in the market, they should consider prescription omega-3 fatty acid products (item 11).” Finally, although the panel has a certain agreement about subtyping specific MDD populations, there are neither “Omega-3 Index” guidance nor pharmacogenomic research data to support personalized medicine for n-3 PUFA treatment.
To conclude, the ISNPR provides the first practice guideline of n-3 PUFAs in depression treatment and emphasizes the importance of accurate clinical diagnosis and measurement-based psychopathological assessments practised in the therapeutic settings. Pure EPA or a combination of EPA and DHA (with net EPA starting from at least 1 up to 2 g/day) for at least 8 weeks are recommended as adjunctive treatment. Monitoring of adverse effects is recommended. In MDD patients with different medical comorbidities, during the perinatal period, or for primary prevention of MDD in specialized populations, there were promising results with good tolerability profiles, but well- designed RCTs with larger sample sizes and longer duration are needed to confirm the efficacy of n-3 PUFAs.
8041 visitors, last modified 25 Sep, 2019,
Printer Friendly
Follow this page for updates
Pages listed in BOTH the categories Depression and Omega-3
- Overweight needed more EPA (4 grams) to fight depression – RCT Aug 2022
- Anxiety, depression, and suicide have recently surged (Note: Vitamin D, Omega-3, and Magnesium help) – May 2022
- Omega-3 did not prevent depression (they failed to reduce Omega-6, which blocks Omega-3) – RCT Dec 2021
- Mental health not helped by vitamin D monotherapy (adding Omega-3 and Magnesium help) – review Nov 2021
- Benefits of Omega-3 plus Vitamin D were additive – RCT Sept 2021
- Depression treatments: diet, exercise, bright light, Vitamin D, B12, Omega-3, Zinc, Music, etc. – May 2019
- Omega-3 helps treat Major Depression – International Consensus Sept 2019
- Mental disorders fought by Omega-3 etc. - meta-meta-analysis Oct 2019
- Omega-3 reduces Depression. Anxiety, Stress, PTSD, etc. – Aug 2018
- Depression treated by Omega-3 (again) – meta-analysis Aug 2019
- Depression after childbirth 5 X less likely if good Omega-3 index – April 2019
- Occupational burnout reduced after 8 weeks of Omega-3 – RCT July 2019
- Anxiety severity reduced if more than 2 grams of Omega-3 – meta-analysis Sept 2018
- Psychotic disorders not treated by Omega-3 when patents take anti-depressants and get therapy – June 2018
- Happy Nurses Project gave Omega-3 for 3 months – reduced depression, insomnia, anxiety, etc for a year – RCT July 2018
- Depression – is it reduced by Vitamin D and or Omega-3 – RCT 2019
- Benefits of Omega-3 beyond heart health - LEF Feb 2018
- Omega-3 improves gut bacteria, reduces inflammation and depression – Dec 2017
- Unipolar depression treated by Omega-3, Zinc, and probably Vitamin D – meta-analysis Oct 2017
- Omega-3 reduces many psychiatric disorders – 2 reviews 2016
- Omega-3 does not consistently treat depression if use small amounts for short time period – review Oct 2016
- How Omega-3 Fights Depression – LEF July 2016
- Depression due to inflammation reduced by Omega-3 (children and pregnant) – Nov 2015
- Depression treated somewhat by Omega-3 (St. John's Wort better) – RAND org reviews 2015
- Depression substantially decreased with Omega-3 – Sept 2015
- Omega-3 for just 3 months greatly reduced psychosis for 80 months – RCT Aug 2015
- Omega-3 prevents PTSD and some mood disorders - Aug 2015
- Omega-3, Vitamin D, and other nutrients decrease mental health problems – March 2015
Meta-analyses of Vitamin D and Depression
- Depression reduced by 8,000 IU of Vitamin D daily – meta-analysis Nov 2024
- Depression 1.6 X more likely if low Vitamin D, taking Vitamin D reduces depression – umbrella of meta-analyses – Jan 2023
- Depression in seniors greatly reduced by Vitamin D (50,000 IU weekly) – meta-analysis June 2023
- Depression reduced if take more than 5,000 IU of vitamin D daily – umbrella meta-analysis – Jan 2023
- Depression reduced if use more than 2,800 IU of vitamin D – meta-analysis Aug 2022
- Depression is treated by 2,000 IU of Vitamin D – 2 meta-analyses July 2022
- Depression treated by 50K IU Vitamin D weekly (but not 1,000 IU daily) – meta-analysis Jan 2021
- Mental disorders fought by Omega-3 etc. - meta-meta-analysis Oct 2019
- Depression less likely if more Vitamin D (12 percent per 10 ng) – meta-analysis July 2019
- Anxiety severity reduced if more than 2 grams of Omega-3 – meta-analysis Sept 2018
- Less depression in seniors taking enough Omega-3 – meta-analysis July 2018
- Unipolar depression treated by Omega-3, Zinc, and probably Vitamin D – meta-analysis Oct 2017
- Depression is associated with low Magnesium – meta-analysis April 2015
- Clinical Trials of vitamin D can have “biological flaws” – Jan 2015
- Slight depression not reduced by adding vitamin D if already had enough (no surprise) – meta-analysis – Nov 2014
- Anti-depression medication about as good as big increase in vitamin D – meta-analysis of flawless data April 2014
- Depression might be reduced by vitamin D – meta-analysis March 2014
- Low vitamin D and depression - Study and meta-analysis, April 2013
- 2X more likely to be depressed if low vitamin D (cohort studies) - Meta-analysis Jan 2013
Download the PDF from VitaminDWiki
Table 1. ISNPR practice guidelines for n–3 PUFAs in the treatment of MDD
Major depressive disorder (MDD) is a complex mental illness with unmet therapeutic needs. The antidepressant effects of w-3 polyunsaturated fatty acids (n-3 PUFAs) have been widely reported. The subcommittee of the International Society for Nutritional Psychiatry Research organized an expert panel and conducted a literature review and a Delphi process to develop a consensus-based practice guideline for clinical use of n-3 PUFAs in MDD. The guideline focuses on 5 thematic areas: general concepts, acute treatment strategy, depression recurrence monitoring and prevention, use in special populations, and potential safety issues. The key practice guidelines contend that: (1) clinicians and other practitioners are advised to conduct a clinical interview to validate clinical diagnoses, physical conditions, and measurement-based psychopathological assessments in the therapeutic settings when recommending n-3 PUFAs in depression treatment; (2) with respect to formulation and dosage, both pure eicosapentaenoic acid (EPA) or an EPA/docosahexaenoic acid (DHA) combination of a ratio higher than 2 (EPA/DHA >2) are considered effective, and the recommended dosages should be 1-2 g of net EPA daily, from either pure EPA or an EPA/DHA (>2:1) formula; (3) the quality of n-3 PUFAs may affect therapeutic activity; and (4) potential adverse effects, such as gastrointestinal and dermatological conditions, should be monitored, as well as obtaining comprehensive metabolic panels. The expert consensus panel has agreed on using n-3 PUFAs in MDD treatment for pregnant women, children, and the elderly, and prevention in high-risk populations. Personalizing the clinical application of n-3 PUFAs in subgroups of MDD with a low Omega-3 Index or high levels of inflammatory markers might be regarded as areas that deserve future research.
Portions of PDF
Introduction
Major depressive disorder (MDD) affects one tenth of the population and has been the world’s leading cause of disability [1, 2]. MDD is of heterogeneous etiology with multiple contributory biological mechanisms. Pharmacological treatments with the currently available antidepressants, although proven to be effective in treating moderate to severe symptoms in MDD, have only modest effect sizes but various adverse effects [3]. Therefore, to optimize the patients’ outcomes, clinicians need more efficacious and tolerable treatments supported by valid scientific evidence and reliable practice guidelines.
Omega-3 polyunsaturated fatty acids (n-3 PUFAs), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) have drawn clinical attention of medical specialties [4-7]. Several lines of evidence have suggested the efficacy of n-3 PUFAs as a preventive and treatment strategy in MDD, from epidemiological and case-controlled studies [8, 9] to randomized-controlled trials [10-20] and meta-analyses [21-30]. In addition to clinical studies that examine the efficacy [31, 32] and tolerability [33], the mechanisms of n-3 PUFAs’ antidepressant effects have also been rigorously studied. Several key mechanisms have been proposed, including neuronal cell plasticity and neurogenesis, neurotransmitter dysregulation, and neuro-inflammation [6, 34, 35].
Despite the clinical and biological evidence, and empirical experience using n-3 PUFAs as an alternative or adjunctive treatment for MDD, there is a current lack of definitive clinical practice guidelines to assist clinicians in the prescriptive application of n-3 PUFAs for MDD. To address this, an advisory subcommittee from the International Society for Nutritional Psychiatry Research (ISNPR) was formed to provide international consensus- based practice guidelines for the evidence-based prescriptive use of n-3 PUFAs for the treatment of MDD.
Methodology
Overview
The format and content of this practice guideline for n-3 PUFAs were developed through 3 stages, including (1) conducting a literature review on major research findings of n-3 PUFAs in MDD treatment; (2) generating a clinical guideline questionnaire and obtaining consensus through a 2-round, web-based modified mini-Delphi survey; and (3) optimizing the guidelines from consensus meetings with international experts.
Literature Review and Search Strategy
We had the first preparatory meeting held by a subcommittee under the auspices of 7 key members from the ISNPR in November 2017. ISNPR is an international collaboration of academics advocating recognition of various nutrients as crucial determinants of both physical and mental health, and the positional statements had been published elsewhere [36, 37].
A systematic review of published literature was conducted with an emphasis on randomized controlled trials (RCTs), systematic reviews and meta-analyses, focusing on the quality of evidence and the implications. Observational studies, physiological or preclini- cal studies in nonhuman subjects, and ongoing trials would also be reviewed as only supportive evidence.
The subsequent literature review was conducted via PubMed and from the Cochrane Database from inception to May 29, 2019, by the terms “omega-3 polyunsaturated fatty acids,” “n-3 PUFAs,” “major depressive disorder,” and “treatment.” Articles retrieved from these searches and relevant references cited in those articles were also reviewed. High-quality articles published in English, Chinese, and Japanese were included for preliminary review. The outline of the review was drafted by 4 core authors (T.G., D.M., J.S., and K.-P.S.), and then all the authors examined cross-referencing, identified missing important studies, revised the draft of the review, and discussed through subsequent online conferences.
The evidence was categorized using a modified format of the 2011 Levels of Evidence grading system developed by the Oxford Center for Evidence-Based Medicine. Level 1 evidence derives from meta-analyses and systematic reviews of narrow confidence interval (CI) or little conflicting results. Level 2 evidence includes appropriately designed RCTs. Smaller and lower-quality RCTs and nonrandomized controlled cohort/follow-up studies are considered level 3 evidence. Case reports and case series were considered and discussed in the expert consensus meeting to formulate level 4 evidence.
Guideline Questionnaire and Delphi Survey
Four core authors (T.G., D.M., J.S., and K.-P.S.) had an initial online meeting in September 2018 to confirm the evidence obtained from the review and to discuss the major themes and prescriptive issues that could be assessed using a survey (and codified in a resultant consensus guideline). Previous guidelines about n-3 PUFAs and MDD, including using n-3 PUFAs in the treatment of cardiovascular diseases by the American Heart Association, were included as references and discussed to facilitate the formation of the questionnaire [38-40]. After internal correspondences, the draft questionnaire consisted of 20 questions covering 5 major themes: (1) general concepts, (2) acute treatment strategy, (3) depression recurrence monitoring and prevention, (4) special populations, and (5) safety issues.
A Likert scale (0-10) was used for the 19 closed questions (0 = fully against, 10 = fully agree). Questions that scored a predefined consensus level (7.0/10) were included into the guidelines, questions that scored between 5.1 and 6.9 were discussed while questions with scores lower than 5.0 were allocated into a proposed second round Delphi survey with inverse questions. The questionnaire was then tabulated in a web-based survey platform (Survey Monkey) for online voting.
Consensus Meetings
There were 14 experts who participated in the Delphi survey conducted in October 2018. The panellists were selected mainly according to the h-index of the Web of Science database, under the topic of “depression and omega-3” with time span until the end of September 2018 and completed with purposive snowball sampling among active ISNPR members in this topic.
In the online consensus meetings, the panellists focused on confirming the affirmed statements for inclusion and discussing the 3 questions that did not reach the consensus level. After the meeting, the results from the literature review, together with recommendations and the feedback from the survey and meeting, were synthesized into the final version of the official practice guidelines.
Results
Diagnosis and Measurement-Based Care
The panel consensus emphasizes the importance on diagnosis-based clinical interview and measurement- based depression assessments to validate the accurate diagnosis and psychopathology severity of MDD in the clinical setting [41]. Indeed, when the antidepressant efficacy of n-3 PUFAs is rigorously studied, the pooled effects favor n-3 PUFAs over placebo mainly in RCTs with clinician interview-based diagnosis, rather than in trials including subjects with subclinical depressive symptoms screened by questionnaire [23, 29].
Disease Stages
Acute Stage of MDD
The efficacy of small to moderate effect sizes with good safety profiles has been reported in several well-designed RCTs with MDD patients in the acute stage [10-12, 20]. Further, the pooled results from the RCTs for MDD adults (nonpregnant and no-children populations) support the use of n-3 PUFAs for “adjunctive” treatment rather than n-3 PUFA monotherapy (level 1) [24, 25, 30, 42, 43].
Recurrent MDD and Maintenance Treatment
Patients with MDD usually require maintenance treatment after the acute depressive episode. However, we identified no studies having enough follow-up duration to address the prevention effects for recurrent MDD. Therefore, current evidence is not adequate to either support or refute the prescription of n-3 PUFAs as longer- term maintenance therapy or for recurrent major depressive episodes. However, considering that most antidepressant medication might be associated with higher dropout rates as compared to placebo [3], the advantage of tolerability profiles of n-3 PUFAs might suggest its potential in maintenance treatment (level 2).
Treatment Strategy (Monotherapy, Augmentation, or Acceleration)
The panel endorses the application for augmentative therapy of n-3 PUFAs because cumulative evidence favors the efficacy of n-3 PUFAs over placebo with the enhanced antidepressant effects clearly shown in meta-analyses (level 1) [24, 25, 30, 42, 43]. Furthermore, in one metaanalysis investigating the time points when n-3 PUFAs were added to antidepressants [42], the results showed beneficial effects in either adding n-3 PUFAs at the beginning of the treatment (as an accelerating agent) or adding them at the time when the standing antidepressant was inadequate (i.e., as an augmentative agent; level 1).
Our literature review revealed only a few studies assessing n-3 PUFAs as a monotherapy for MDD. Monotherapy with n-3 PUFAs may have therapeutic benefits in children [44] and pregnant women [14] with depression. However, Mischoulon et al. [45] revealed that neither EPA nor DHA monotherapy were superior to placebo in MDD adults. In another study, patients with post- myocardial infarction were treated with 460 mg EPA and 380 mg DHA for 12 months as monotherapy, and there was also no significant effect in favor of n-3 PUFAs [46]. Therefore, current evidence is inadequate overall of using n-3 PUFAs as monotherapy for MDD, and further RCTs are needed to provide support for or against the use of n-3 PUFAs as a monotherapy for adult MDD (nonpregnant and no-children) patients (level 2).
Table 1. ISNPR practice guidelines for n-3 PUFAs in the treatment of MDD (at top of page)
Dosing, Ratio, and Duration
The functional status of n-3 PUFAs in an individual can be influenced by many factors like dietary patterns, gender, age, and metabolic functions. Therefore, it is impossible to predict the absolute effects of a fixed dose n-3 PUFA supplementation in MDD individuals. In addition, there is no RCT investigating clinical dosing of n-3 PUFAs based on individual dietary or blood level differences.
According to the available RCT results, the panel advises that the starting dose should be at least 1 g of net EPA in a pure EPA form or in an EPA/DHA combination (ratio higher than 2). If well tolerated, the dose could be titrated up to at least 2 g of net EPA daily in 2-4 weeks for partial responsiveness (level 1) [21,22, 24, 25, 27-30, 42, 43]. The panel also reaches a unanimous consensus on the recommendation of EPA/DHA ratio of >2:1 to be crucial to n-3 PUFAs’ antidepressant effects (level 1). Indeed, this conclusion is supported by most of the meta-analyses that the higher ratio of EPA, the better the therapeutic outcome, while DHA as main component had no detectable pooled effects on the MDD symptoms [21,23-25, 29].
There was insufficient data to conclude the optimal duration of n-3 PUFA supplementation. The duration of most RCTs varies from 4 to 16 weeks. Due to the length of time needed for n-3 PUFAs to be incorporated into brains, and for the downstream neuroplastic effects and anti-inflammatory actions, the panel endorses a prescriptive guideline of at least 8 weeks. Clinicians are advised to check the quality of the n-3 PUFA prescription of nonresponders, and evaluate the response and the tolerability profiles for an optimal duration (level 2) [24, 25, 27].
Safety and Adverse Effects
One recent meta-analysis comprehensively examined the adverse effects reported when using prescribed n-3 PUFA products in various clinical populations. n-3 PUFAs were found to be associated with higher rates of mild gastrointestinal symptoms (such as fishy taste, belching, and nausea), less in skin abnormalities (such as eruption and itchiness), and no serious adverse effects [33]. Another meta-analysis using adverse effects as one of the primary outcomes reported that, despite poor data quality, the numbers of individuals experiencing adverse events were similar in intervention and placebo groups and were negligible (level 1) [30]. The risk of theoretical adverse effects of excessive bleeding did not exist, and current evidence suggests that under concurrent usage of antiplatelet or anticoagulant agents, doses up to 4 g of n-3 PUFAs daily are not associated with an increased risk of major bleeding [47]. Although both a recent meta-analysis focusing on pregnancy [48] and the other focusing on perioperative periods [49] support similar safety profiles compared with placebo in general and in bleeding-related adverse events, and most in vivo studies do not suggest that n-3 PUFAs would impact either platelet aggregation or adhesion in healthy subjects [50], we recommend shared decision-making discussions between patients and clinicians on bleeding-related hematological examinations, especially if the patient has underlying fibrinogen dysfunction or is taking antiplatelet or anticoagulant agents.
Laboratory measurements have revealed that n-3 PUFAs (compared to placebo) may cause higher fasting blood sugar, glutamate pyruvate transaminase, low-density lipoprotein cholesterol and blood urea nitrogen, and lower hemoglobin and hematocrit levels. Although the mean differences were relatively small, and may have only statistical rather than clinical importance, the panel recommends that, when using higher doses, these biomarkers may need to be monitored (level 1). It is interesting to note that in this meta-analysis, combining EPA and DHA was associated with higher rates of the aforementioned adverse effects, compared with EPA alone [33]. The monitoring profiles are summarized in Table 2.
Discussion
To our knowledge, this is the first international research society consensus-based practice guideline for clinical use of n-3 PUFAs in MDD. The guideline includes 12 clinical recommendations and is well summarized in Table 1 based on the items which reached consensus levels. In general, the recommendations focus on 5 thematic areas: general concepts, acute treatment strategy, recurrence and prevention, special populations, and safety. The guideline did not include, yet discussed in the previous section Results, the 3 items of our expert survey that did not reach consensus levels.
The panel emphasizes the importance of accurate clinical diagnosis and measurement-based psychopathological assessments practised in the therapeutic settings when recommending n-3 PUFAs in depression treatment. As cumulative evidence favors the efficacy of n-3 PUFAs as an adjunctive use on top of antidepressant medication, the attitude of prescribing n-3 PUFAs should not be based on antimedication.
As meta-analyses reveal only small but statistically significant effects, there are always critical debates on the benefits of n-3 PUFAs for MDD. The estimated effect sizes (standardized mean differences between n-3 PUFAs and placebo) range from 0.23 to 0.56 (with wide CI) in treating DSM-defined MDD patients in 3 recent metaanalyses [24, 29, 30]. However, small effect sizes are also reported for all kinds of antidepressant drugs compared to placebo, with the standardized mean differences between 0.30 and 0.47 (with narrower CI) [3, 66, 67]. Till now, the scientific evidence and clinical opinions do not support any particular treatment based on efficacy differences. Although n-3 PUFAs would not be regarded as “a new antidepressant,” when the clinicians consider taking the differences in adverse events and patients’ preference into consideration, n-3 PUFAs would be potentially an applicable alternative choice of treatment [68].
Three practical strategies could be considered for the unmet needs in depression treatment as every single recommended therapy only has small effects: “an open- minded attitude to integrative intervention,” “the application of personalized medicine,” and a “shared decision making process based on balanced information to enhance treatment adherence.”
First, the integrative intervention is often criticized for overpromotion on efficacy and safety and for nonregulation of product quality and malpractice by trustworthy authorities. This panel consists of the most cited experts in the fields and aims to provide an evidence-based empirical practice guideline to help clinicians to have a more open-minded attitude to recommend n-3 PUFAs to depressed patients. For other “nonpatentable” interventions with sound clinical and preclinical evidence (e.g., exercise [69], mindfulness [70, 71], or acupuncture [72]), we need more well-established expert consensus and practice guidelines supported by trustworthy authorities for the same reasons.
The second important strategy to increase the treatment effects is subtyping patients into more homogeneous subgroups, as MDD populations can be extremely heterogenous based on current diagnostic systems [73]. Rapaport et al. [18] published an interesting RCT and demonstrated that EPA monotherapy was more effective than the placebo or DHA in patients with elevated markers of inflammation. Specifically, the overall treatment group differences were negligible (ES = -0.09 to -0.13) among MDD patients without stratifying. However, subjects classified with “high” inflammation improved more on EPA than placebo (ES = -0.39 to -1.11 from any one marker to 4-5 markers) or DHA (ES = -0.60 to -1.10 from any one marker to 4-5 markers). Furthermore, EPA-placebo separation increased with increasing numbers of markers of high inflammation. In other words, employing biomarkers of inflammation indeed facilitated identification of a more homogeneous cohort of subjects with MDD who would respond better to n-3 PUFA antidepressant treatment [32]. Therefore, this current practice guideline specifies subgroups of MDD for n-3 PUFAs, including perinatal depression, childhood depression, and MDD with low-grade inflammation or comorbid obesity.
Finally, it has been noted that a shared decision-making process may enhance patients’ adherence and satisfaction to treatment, especially in chronic illnesses [74]. As MDD is often a long-lasting, relapse-remitting illness, assessing the patient’s attitudes toward treatment, integrating information regarding responsiveness, vulnerability, comorbidity and important psychosocial and contextual factors, rather than providing a reductionistic view of treatment options, may allow the patients to actively participate in the therapeutic settings and subsequently improve the adherence and satisfaction toward the treatment [75-78]. In this guideline, we tried to address all the relevant clinical aspects when considering n-3 PUFAs for MDD patients, and it is important to emphasize that “the process” of discussing the information can be as important as the information itself.
Despite several meta-analyses and independent clinical trials which have shown that n-3 PUFAs were more effective than placebo, the meta-analyses from 2 groups failed to detect antidepressant effects of n-3 PUFAs [22, 79]. In fact, the main reasons of inconsistent results from metaanalyses in n-3 PUFA trials are due to common methodological flaws such as pooling heterogeneous clinical trials, applying nonstandardized diagnostic procedures and unreliable outcome measurements, and implementing improper intervention methods [29, 80], which are rarely found in high-quality clinical trials conducted by pharmaceutical companies. In the first meta-analysis with negative findings [22], the authors included a clinical trial [81] enrolling individuals according to self-rating scales, but not structured interview, and in nonclinical settings that weighted up to 31.7% of the whole pooled estimation. In another meta-analysis showing negative outcomes [79], one single 43.3%-weighted clinical trial was included [82], which not only applied no appropriate tools for clinical diagnoses or assessments, but also defined its n-3 PUFA intervention as “advising” subjects to “eat more fish.” Obviously, the methodological flaws undermined the possibility to detect the small signals in clinical trials for n-3 PUFAs’ antidepressant effects. And if the problematic RCTs were taken away, all the meta-analyses from independent groups with well-conducted designs showed positive results [23-25, 27, 29, 83].
Some limitations need to be taken into account when applying this guideline in clinical settings. First, 3 items did not reach consensus levels: (1) “n-3 PUFAs are one of the potential monotherapies for adult MDD”; (2) “pure EPA is more recommended than EPA/DHA (>2) combination”; and (3) “n-3 PUFAs are considered similarly effective for recurrent MDD.” Therefore, we concluded that the evidence is inadequate overall of using n-3 PUFA prevention of MDD recurrence or as a monotherapy. In addition, the consensus describes that “the pure EPA form or the EPA/DHA combination could both be effective.” Second, we should be aware of the aggregated mild treatment effects in meta-analyses and the inconsistence in some of the RCTs. In addition to general methodological flaws mentioned previously, the other main reasons for inconsistent findings from several RCTs include insufficient statistic powers due to small sample sizes, poor quality of product preparations, and inadequate dosage or ratio of EPA in the n-3 PUFA supplementations. It is very important to note that the clinical trials using DHA-predominant formulations are consistently ineffective [55, 56, 81, 84-87]. Therefore, this guideline includes: “Both pure EPA and EPA/DHA (ratio >2:1) combinations are effective as a potential treatment of MDD (item 4).” Third, unlike medication, most n-3 PUFA supplementary products are not strictly regulated by government regulation. Therefore, this guideline recommends that “If clinicians are not familiar with high-quality n-3 PUFAs in the market, they should consider prescription omega-3 fatty acid products (item 11).” Finally, although the panel has a certain agreement about subtyping specific MDD populations, there are neither “Omega-3 Index” guidance nor pharmacogenomic research data to support personalized medicine for n-3 PUFA treatment.
To conclude, the ISNPR provides the first practice guideline of n-3 PUFAs in depression treatment and emphasizes the importance of accurate clinical diagnosis and measurement-based psychopathological assessments practised in the therapeutic settings. Pure EPA or a combination of EPA and DHA (with net EPA starting from at least 1 up to 2 g/day) for at least 8 weeks are recommended as adjunctive treatment. Monitoring of adverse effects is recommended. In MDD patients with different medical comorbidities, during the perinatal period, or for primary prevention of MDD in specialized populations, there were promising results with good tolerability profiles, but well- designed RCTs with larger sample sizes and longer duration are needed to confirm the efficacy of n-3 PUFAs.
| 8041 visitors, last modified 25 Sep, 2019, |